Biomedical Data Science Hub
Date stamp: 7.21.2025
Jump to: About | People | Policies | Roles & Responsibilities | Resources & Training
A comprehensive review of the BDS Hub's Standard Operating Procedures for Statistical Activities has been made available as a resource for the following activities and needs:
- Communicating our operating principles and procedures to our Dell Med collaborators,
- Guiding the articulation of project methodological needs, roles, and responsibilities—both during project conceptualization (e.g., in the context of a grant application) and throughout project implementation,
- Foster continuity and consistency in methodology across projects and staff,
- Onboarding new BDS Hub staff.
A brief summary of the most common topics is given below; please refer to the official SOP for further details and information.
About: Mission, Areas of Inquiry, Principles
Mission
The mission of the BDS Hub is to serve Dell Medical School as a scholarly resource for collaborative application of quantitative research methods, as well as development of new such methods, in support of Dell Medical School’s missions in areas of biomedical, clinical, and population health investigation and innovation.
Areas of Inquiry
The primary areas of methodological activity include:
- Clinical biostatistics, including clinical trials, health services research, and population health
- Clinical research informatics
- Bioinformatics
And, as needed:
- Statistical genetics and genomics
- Imaging analysis and informatics
Principles Driving the BDS Hub
Dell Medical School has the opportunity to rethink how health care is delivered, thus we should also rethink how data drives that process.
The BDS Hub seeks to employ and deploy data science faculty and staff geared towards collaborative scholarship, willing to invest in others’ projects via a long-term collaborative process, while seeking ways to advance their own scholarly agenda. As such, we expect to be involved soup-to-nuts: early on, from the conception of a project, all the way through the submission of the last manuscript. The goal is to work with Dell Med investigators to foster the evolution of their research careers that is mutually beneficial in terms of creative, rigorous, and funded scholarship that is well-rounded and minimizes missteps and lost time and effort.
Key principles:
- Ground activity in modern principles of data science
- Soup-to-nuts collaborative activity in investigations from project inception to final report, with PhD level BDS Hub members serving as Co-I, and MS level BDS Hub members serving as Biostatistician or Analyst
- Develop novel quantitative methodologies independently initiated or stimulated by collaborative work
- Foster scientific integrity for DMS research projects
- Employ state-of-the-art quantitative methods
- Leverage existing strengths on the broader UT campus
- Serve as a methodological concierge to new projects, and pipeline to methodological resources elsewhere in DMS or across UT more broadly to support such project
Please review our policies listed below, or, for more details, see our current Strategic Plan.
Please contact aubrey.hooser@austin.utexas.edu with questions or if you would like some human interaction around your project/problem.
Jump to: About | People | Policies | Roles & Responsibilities | Resources & Training
People
Faculty and Staff
- Paul Rathouz, PhD: Director, Biomedical Data Science Hub; Professor, Department of Population Health, paul.rathouz@austin.utexas.edu
- Thomas Achia, PhD: Senior Biostatistician, thomas.achia@austin.utexas.edu
- Nazan Aksan, PhD: Senior Biostatistician, nazan.aksan@austin.utexas.edu
- Haresh Rochani, PhD: Senior Biostatistician, haresh.rochani@austin.utexas.edu
- Patrick Chang, MS: Biostatistician II, patrick.chang@austin.utexas.edu
- Arnold Kuk, MS: Biostatistician II, arnold.kuk@austin.utexas.edu
- Sina Sanei, MS: Biostatistician II, sina.sanei@austin.utexas.edu
- Aubrey Hooser, MA: Program Administrator, aubrey.hooser@austin.utexas.edu
Affiliate Faculty
- Jeanne Kowalski-Muegge, PhD: Associate Director of Cancer Clinical Genomics, LIVESTRONG Cancer Institutes; Professor, Department of Oncology, jeanne.kowalski@austin.utexas.edu
- Elizabeth Matsui, MD, MHS: Director of Clinical and Translational Research; Professor, Department of Population Health; Professor, Department of Pediatrics, ematsui@utexas.edu
Jump to: About | People | Policies | Roles & Responsibilities | Resources & Training
Policies for BDS Hub Collaborative Activity
The BDS Hub Intake Prioritization Framework establishes a structured process for prioritizing project requests, ensuring that resources are allocated efficiently to support high-impact and funded research initiatives. By setting clear expectations for scheduling, effort allocation, funding, and authorship, this framework enhances collaboration and maximizes research success. This SOP serves as a guide for investigators and research teams seeking BDS Hub support, ensuring consistency, transparency, and adherence to best practices in project engagement.
Intake and Engagement Prioritization
This SOP applies to all projects and investigators requesting BDSHub support, including grant applications, preliminary analyses for funding proposals, manuscript preparation, and faculty research projects. It also defines the expectations for effort allocation, funding recovery, and authorship recognition. BDSHub prioritizes engagement based on funding potential and research impact. Project requests are categorized as follows:
- Top Priority (Top 1)
• Grant or funding applications. - High Priority (Top 2)
• Analysis on currently funded research projects.
• Work leading to grant/contract submissions (e.g., preliminary analyses for grants, manuscript preparation). - Medium Priority (Top 3)
• Junior faculty start-up projects. - Not Formally Prioritized: Feasibility will be assessed on a case-by-case basis
• Unfunded work on unfunded projects.
• Unfunded work on funded projects.
Note: If another UT or Dell Medical School unit wants to fund work internally, then the Biomedical Data Science Hub considers that to be “funded” and it would not figure into this calculus.
Scheduling
BDS Hub engagement includes both long-term project collaboration (e.g., data collection and analysis) and shorter-term, focused efforts (e.g., grant writing, manuscript development). To ensure timely and effective support, the following lead times are recommended:
- Projects Involving Primary Data Collection
- Requests should be submitted 8–10 weeks before the due date at Office of Sponsored Projects (OSP) deadline.
- Alternatively, a case-by-case approach may be used to determine a mutually agreeable timeline based on project complexity and scope.
- Projects Involving Only Secondary Data (e.g., Analysis of Existing Data)
- Requests should be submitted 6–8 weeks before the due date at OSP.
- Case-by-case scheduling may apply depending on project needs and resource availability.
Note: Grant writing and manuscript preparation are treated as distinct, time-sensitive project phases. While often part of a larger research effort, these components are considered standalone workstreams for planning purposes and should be scheduled accordingly.
Effort and Funding
- The estimated percentage of effort required for each project should be included in the grant budget.
- BDSHub funds will cover preparatory work for projects that lead to funding applications.
- For sponsored projects, salary for BDSHub effort should be recovered at the same level as effort expended.
- BDS Hub effort is estimated, not negotiated. It depends on the needed work. The required effort for each project will be determined case-by-case, ensuring alignment with available resources.
- BDS Hub staff estimate effort levels depending on the needed scope of work, not the PI.
- As the researchers conducting the data science (biostatistics or bioinformatics) components of a project, those faculty and staff are in the best position to make an accurate estimate of the effort required, perhaps, in the case of staff, with the help of their supervisor.
- In particular, it is not appropriate for the PI on a collaborative project to do this estimation in isolation of coordination with BDS Hub leadership.
Authorship and Acknowledgement
Grant/Contract Applications: On collaborative projects and grant/contract applications, MS-level BDS Hub staff should be included as research staff (e.g., "Biostatistician") and PhD-level BDS Hub faculty and staff should be included as Co-Investigator(s) (Co-I[s]); exceptions are rare. If this is not possible due to terms of a particular any proposal/application, it is the responsibility of the PI to bring this to the Director's attention at intake.
Authorship Guidelines: All personnel who contribute meaningfully to project development or execution should be considered for authorship (see, The International Committee of Medical Journal Editors). This includes contributions to preliminary data manuscripts (e.g., scholarly input during early-stage development) as well as involvement in general research activities, such as data analysis, writing, reviewing manuscript content, and other significant project roles.
When BDS Hub faculty and staff perform work on unfunded scholarly products (e.g., peer-reviewed manuscripts or public technical reports), please include the acknowledgement: "[Insert BDS Hub Staff name(s)]'s effort on this project was supported by core funds of the Dell Medical School at the University of Texas at Austin."
Collaboration & Documentation
BDS Hub will work closely with Principal Investigators (PIs), research teams, and relevant stakeholders to ensure smooth project execution.
Modifications to project timelines, funding plans, or authorship expectations should be communicated to all involved parties; and in particular to the BDS Hub Program Administrator.
Jump to: About | People | Policies | Roles & Responsibilities | Resources & Training
Statistical Roles
The expertise of the BDS Hub faculty and staff may be utilized for certain roles according to the scope and needs of a particular project:
- Study Statistician (StuStat): responsible for the successful design, implementation, analysis, and dissemination of a research study, thereby ensuring the scientific integrity and statistical rigor of the study as well as the accurate representation of findings.
- Data Coordinating Center (DCC): responsible for overseeing the study's data management and quality assurance processes, particularly in clinical and epidemiological studies. The DCC may function independently or in combination with the Study Statistician (StuStat) role, depending on project structure and staffing.
- Statistical Data Analysis Center (SDAC): The SDAC plays a critical role in ensuring the highest level of study rigor, participant safety, and unbiased statistical oversight in support of the Data and Safety Monitoring Board (DSMB) and in collaboration with the DSMB, Study Statistician (StuStat), and other study personnel.
For a detailed review of these Roles and their respective responsibilities and and information regarding Data Management, please see the BDS Hub's Standard Operating Procedures for Statistical Activities.
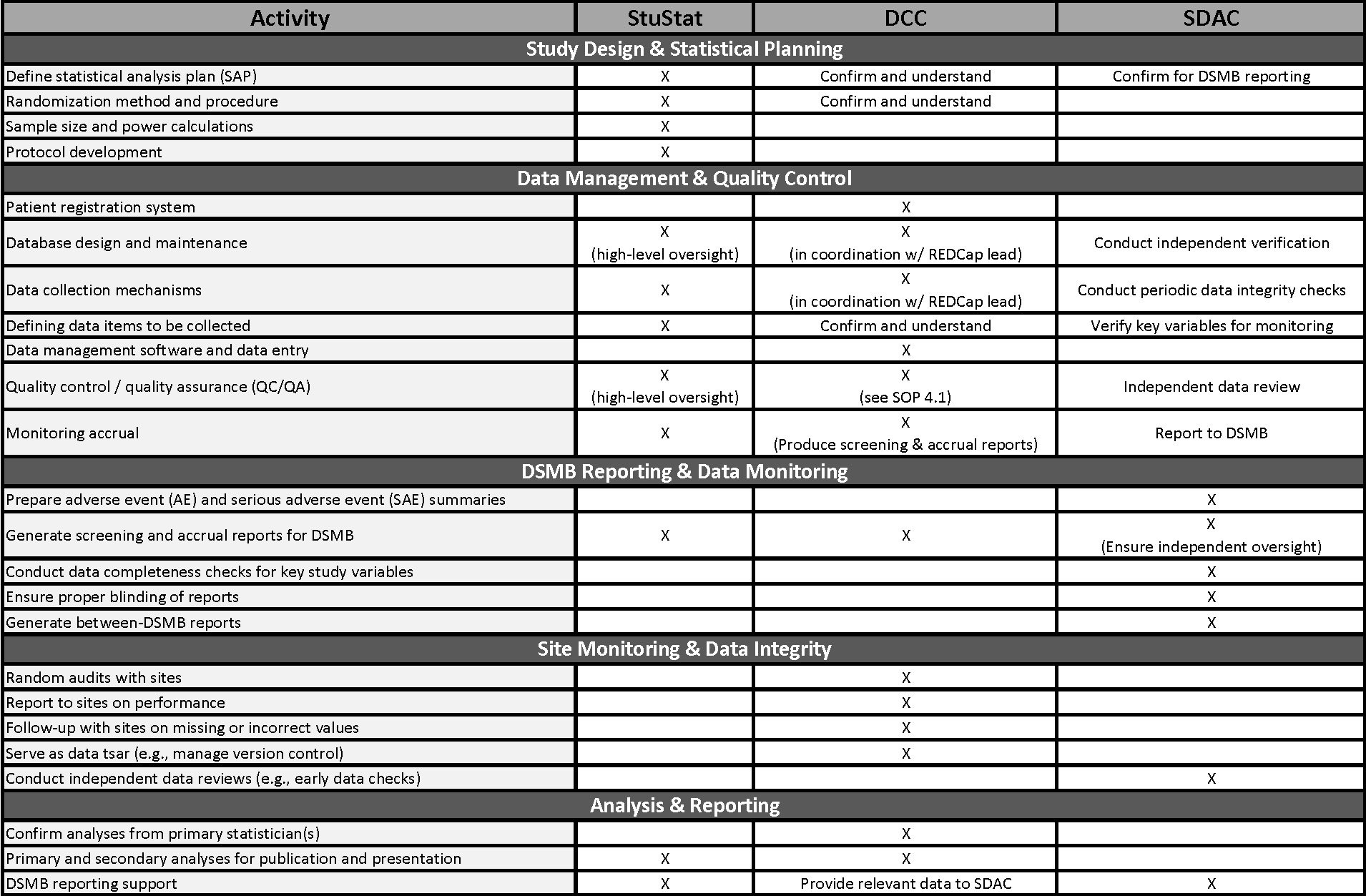
Bridging Statistical Roles: Study Statistician, DCC, and SDAC
This section summarizes and consolidates the statistical roles in primary data collection studies, including intervention trials and observational studies, with a specific focus on the interaction between the Study Statistician (StuStat), the Data Coordinating Center (DCC), and the Statistical Data Analysis Center (SDAC). These roles ensure the highest level of data integrity, participant safety, and unbiased statistical oversight, particularly in studies requiring Data and Safety Monitoring Board (DSMB) reporting.
The statistical team collaborates across multiple functions, with the StuStat primarily responsible for study design and analysis, the DCC for data management and quality control, and the SDAC for independent data monitoring and DSMB reporting:
Additional Considerations for Observational Studies
- A key additional role of the StuStat is that when designing observational studies, we need to assess if such a study is feasible before going down that path.
- This may involve and include power and sample size (PSS) calculations.
- It should involve an assessment of sufficient overlap in the confounder/adjustment space between/among the “treatment” groups, if applicable.
Blinding Considerations
- SDAC personnel may have access to unblinded data for DSMB reporting while ensuring reports remain blinded as needed.
- The StuStat should remain blinded, where feasible, to maintain independence from data monitoring activities.
- The DCC remains blinded until final data collection is complete, and the database is locked.
Coordination with Other Entities
- If the SDAC role is assumed externally, internal teams ensure alignment with institutional policies.
- The DCC and SDAC coordinate closely to ensure accurate accrual tracking and data quality monitoring.
- If BDSHub covers multiple roles (StuStat, DCC, and SDAC), responsibilities will be divided to maintain appropriate independence and oversight.
Jump to: About | People | Policies | Roles & Responsibilities | Resources & Training
Resources & Training
Local Didactic Courses
From Great Idea to Clear Results: A year long course offered in conjunction with Department of Women's Health, Dell Med Office of Research, and UTHealth School of Public Health in Austin for those who could benefit from learning research study design.
Research Nuts & Bolts (offered by the Dell Med Office of Research) is a one-hour monthly forum focusing on topics related to execution of clinical and population research.
Looking for a more specialized course on the UT-Austin campus? Contact the BDS Hub and we will try to help you locate one.
General Texts and Asynchronous Training
The Art of Data Science, by Roger D. Peng, and Elizabeth Matsui. Note you can pay as little as $0 if you decline the lecture videos and choose only the book option.
The Data Science Salon: A Collaborative Learning Experience, by Roger D. Peng, Elizabeth Matsui, and Corinne Keet.
Understanding data and statistics in the medical literature, by Jeffrey Leek, Lucy D'Agostino McGowan, and Elizabeth Matsui.
Useful Articles
Broman, K. W., & Woo, K. H. (2018). Data Organization in Spreadsheets. The American Statistician, 72(1), 2-10.
Leek, J., & Peng, R. (2015). What is the question? Insights, 347(6228) 1314-1315.
Have questions? Please email: aubrey.hooser@austin.utexas.edu.